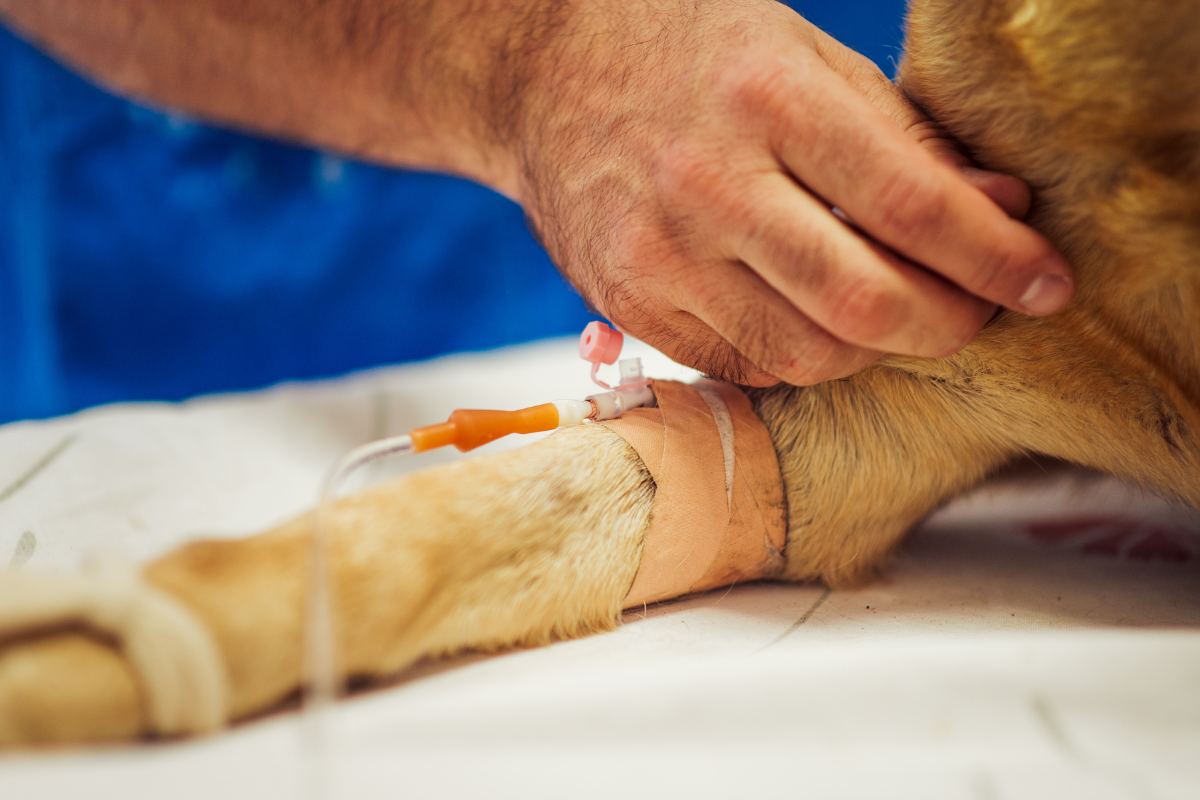
Immunotherapy Combined with a Single Dose of Chemotherapy Shows Improved Outcomes in Dogs with Bone Cancer
Recently, ELIAS Animal Health announced new data on a chemo-immunotherapy approach using the ELIAS Cancer Immunotherapy (ECI®) to treat canine osteosarcoma that demonstrated improved outcomes. The analysis showed that combining the initial cytotoxic effects of chemotherapy with the targeted immune-mediated response stimulated by ECI® has the potential to enhance overall response and survival rates in canine cancer patients. In the study, 14 dogs received one dose of carboplatin followed by ECI® initiated 21 days later. This treatment group showed the best response, with a 1-year survival rate of 71% compared to 21% in 14 matched control dogs that received four doses of carboplatin only. See the data>> An updated in-depth analysis of this combination approach with additional data will be presented at the 2025 Veterinary Cancer Society (VCS) Annual Conference in Salt Lake City, Utah. Jeffrey N Bryan, DVM, MS, PhD, DACVIM(O), who presented the first analysis at the 2025 [...]