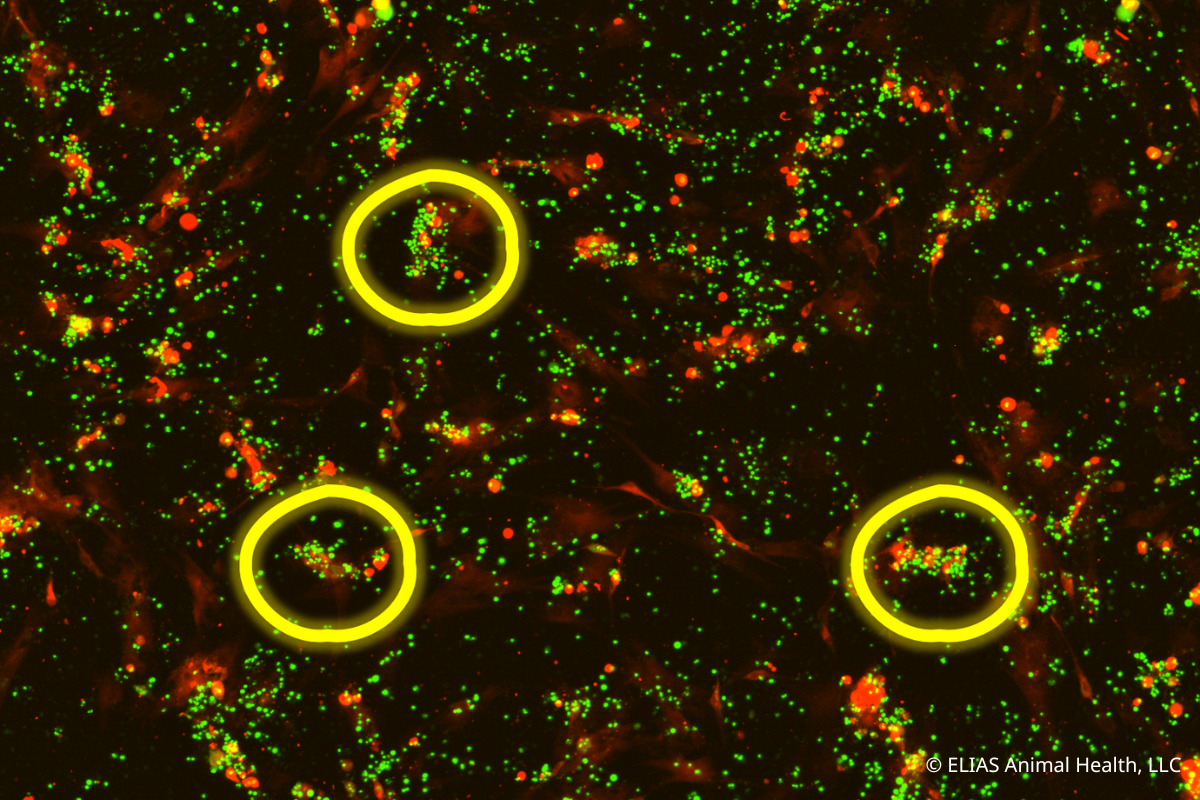
ELIAS Animal Health Research Demonstrates Cancer-Killing Capabilities of Its Activated T Cell Immunotherapy
In vitro study of the ELIAS Cancer Immunotherapy (ECI®) showed personalized T cell immunotherapy initiated a significant immune response against target cancer cells. OLATHE, Kan., August 10, 2022 -- ELIAS Animal Health recently presented new mechanism of action data for the ELIAS Cancer Immunotherapy (ECI®) at the 2022 American College of Veterinary Internal Medicine Forum. ECI is an adoptive cell therapy that stimulates a patient’s immune system to recognize and attack cancers. ECI uses a personalized vaccine made from a patient’s own cancer cells to “prime” the immune cells to recognize the cancer. These primed immune cells—which are collected from the patient through a procedure called apheresis—are activated and expanded ex vivo for reinfusion into the patient, where they travel to the cancer cells and attack them. In vitro study demonstrates how vaccine-primed T cells mount an immune response against cancer cells [...]