News & Updates
Stay in the Know
Don’t miss the latest news and updates from ELIAS Animal Health
ELIAS Animal Health Appoints Chief Revenue Officer; Launches Fundraising Round to Support Commercialization and Development of Innovative Canine Cancer Therapies
Experienced executive will lead launch of the first USDA-approved immunotherapy for the treatment of bone cancer in dogs OLATHE, Kan., April 10, 2024 /PRNewswire/ -- ELIAS Animal Health, a leading companion animal cancer therapeutics company, today announced the appointment of Brian Segebrecht as Chief Revenue Officer in anticipation of the impending product approval of the ELIAS Cancer Immunotherapy (ECI®) in Q4. Segebrecht joins the ELIAS leadership team after recently leading Sentrx Animal Care as CEO to a point of exit in 2023. At ELIAS, he will lead the commercialization of the company’s first-in-class adoptive cell therapy, as well as drive new [...]
USDA Agrees Clinical Trial Data for the ELIAS Cancer Immunotherapy (ECI®) Demonstrates Reasonable Expectation of Efficacy for the Treatment of Bone Cancer in Dogs
First-in-class adoptive cell therapy for treatment of osteosarcoma, a deadly form of bone cancer in dogs OLATHE, Kan., Jan. 17, 2024 /PRNewswire/ -- ELIAS Animal Health, a leading companion animal cancer therapeutics company, today announced the U.S. Department of Agriculture Center for Veterinary Biologics has determined that the data from the company’s ECI-OSA-04 pivotal combined safety and efficacy study demonstrated a reasonable expectation of efficacy, a critical milestone in the licensure pathway. Cancer is the leading cause of death in dogs over the age of two and represents a significant unmet medical need in veterinary medicine. This two-arm field safety [...]
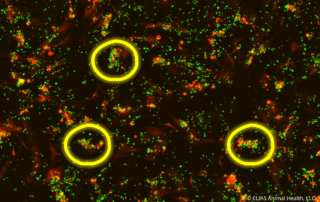
ELIAS Animal Health Research Demonstrates Cancer-Killing Capabilities of Its Activated T Cell Immunotherapy
In vitro study of the ELIAS Cancer Immunotherapy (ECI®) showed personalized T cell immunotherapy initiated a significant immune response against target cancer cells. OLATHE, Kan., August 10, 2022 -- ELIAS Animal Health recently presented new mechanism of action data for the ELIAS Cancer Immunotherapy (ECI®) at the 2022 American College of Veterinary Internal Medicine Forum. ECI is an adoptive cell therapy that stimulates a patient’s immune system to recognize and attack cancers. ECI uses a personalized vaccine made from a patient’s own cancer cells to “prime” the immune cells to recognize the cancer. These primed [...]
ELIAS Animal Health Expands Scientific Advisory Board
Nov. 23, 2021 — ELIAS Animal Health, a clinical stage development company advancing novel treatments for cancer in companion animals, announces the appointment Zachary Wright, DVM, DACVIM (Oncology) and Amanda Foskett, DVM, DACVIM (Oncology) to its scientific advisory board. “I am pleased to add two veterinary oncologists to our advisory board," said Tammie Wahaus, CEO of ELIAS Animal Health. "Drs. Wright and Foskett bring an invaluable perspective on the advancement of animal cancer treatments.” Dr. Wright is the Medical Director at Animal Diagnostics Clinic at VCA Hospitals in Dallas, Texas. He received his veterinary medical degree from Texas A&M University [...]
ELIAS Animal Health In-Licenses Oncolytic Vaccinia Virus Treatment for Pet Animals from Genelux Corporation
Agreement grants ELIAS Animal Health the worldwide right to development and commercialization of V-VET1 for the diagnosis, prevention and treatment of cancer in veterinary medicine Nov. 18, 2021 -- Genelux Corporation, a clinical-stage immunotherapy company, recently announced a licensing agreement with ELIAS Animal Health for V-VET1, its clinical-stage animal health product candidate. V-VET1 is a vaccinia viral strain which selectively replicates in cancer cells causing cell death (apoptosis). ELIAS Animal Health is planning clinical trials to evaluate and develop V-VET1 as a potential new immunotherapy option for veterinary oncologists. “We are thrilled to announce our exclusive license for V-VET1 to help [...]
ELIAS Animal Health Launches Clinical Trial Of Cancer Immunotherapy Treatment For Canine Oral Melanoma
Olathe, Kan., August 31, 2021 – ELIAS Animal Health, a clinical stage development company advancing novel treatments for cancer in companion animals, today announced the initiation of a pilot study of its adoptive T cell therapy combined with surgery and radiotherapy, if indicated, for dogs diagnosed with oral malignant melanoma, a highly metastatic disease and the most common form of oral cancer seen in dogs. Melanoma is widely believed to be chemo-resistant and in this study the adoptive T cell therapy will be administered instead of chemotherapy. This clinical trial will enroll dogs that have been newly diagnosed with oral melanoma [...]
Long-term bone cancer survivor and ECI® patient featured on local news
Ruby the Greyhound was diagnosed with osteosarcoma in December 2016 and began the ELIAS cancer immunotherapy (ECI®). Today, Ruby is thriving and her story was recently featured on a Kansas City local news channel. Learn more about Ruby, one of five long-term survivors from the ELIAS preliminary study. Read the story
ELIAS Animal Health Expands Clinical Trial of Immunotherapy Treatment for Cancer in Dogs
Ten sites now participating in trial of alternative treatment to chemotherapy for canine osteosarcoma Olathe, Kansas, Nov. 12, 2020 – ELIAS Animal Health, a clinical stage development company advancing novel treatments for cancer in companion animals, announces that 10 investigation sites across the U.S. are now participating in the pivotal trial of ELIAS cancer immunotherapy (ECI®). This marks an expansion of the study ELIAS initiated in May to pursue licensure of ECI treatment combined with surgery as an alternative to chemotherapy for dogs diagnosed with osteosarcoma, a deadly form of bone cancer. The geographical areas served by the clinical trial [...]